To begin the lab, a leech was caught and anesthetized in a 20% ethanol solution. This type of solution works as an anesthesia on small creatures that breathe through their skin, however, it doesn't work on vertebrate animals. It is important to use a 20% concentration, as a higher concentration might be harmful.
After the leech was anesthetized, it was pinned dorsal side up on the dissection tray. To guarantee the most optimal laboratory conditions, the leech was pinned through the anterior and posterior suckers, stretching the animal in the process.
A cut was then made along the dorsal side of the leech, with careful precision as to not damage any of the underlying structures. The skin along this cut was then pulled back and pinned to expose the innards of the leech. The nervous system has not yet been exposed, as it is located ventrally.
The internal structures of the leech were then removed to expose the nerve cord and nervous system, which is located within the ventral sinus. As the sinuses were exposed, many swellings became visible. These swellings contained segmental ganglia. The next step in the lab process was to expose one of the ganglia, taking extra precaution to not damage the nerve cord or any other attached nerves.
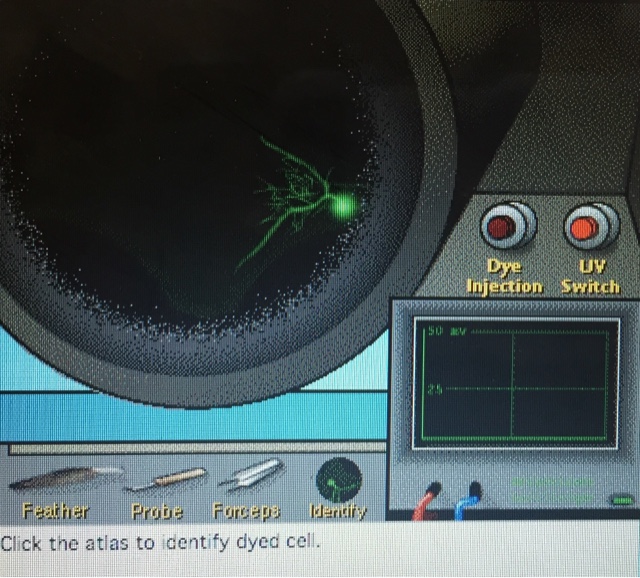
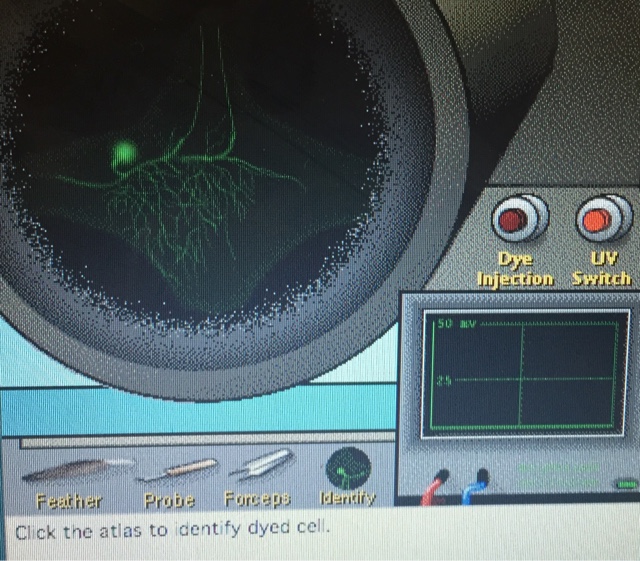
After the ganglion was exposed, we used an electrode to penetrate the ganglion, probing for a cell. Once one was found, a feather, probe, or forceps was used to trigger a response from the nerve cell so that the electrophysiology could be visualized. Once this was completed, fluorescent dye was injected into the cell to visualize the neuron morphology. After the dye was injected, the neuron could be seen under UV light.
After completing the morphology and electrophysiology tests, we were asked to categorize the cell type based on the results compared to the chart below. This process was completed for every cell that was found, and the process can be seen below the categorization chart.
















No comments:
Post a Comment